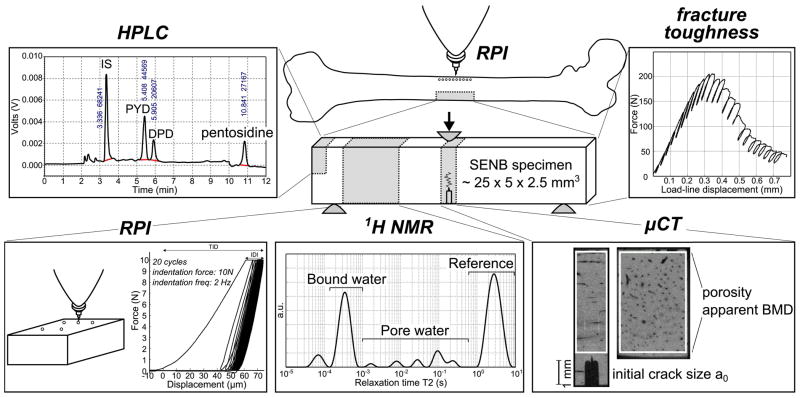
Abstract
Fracture risk does not solely depend on strength but also on fracture toughness, i.e. the ability of bone material to resist crack initiation and propagation. Because resistance to crack growth largely depends on bone properties at the tissue level including collagen characteristics, current X-ray based assessment tools may not be suitable to identify age-, disease-, or treatment-related changes in fracture toughness. To identify useful clinical surrogates that could improve the assessment of fracture resistance, we investigated the potential of 1H nuclear magnetic resonance spectroscopy (NMR) and reference point indentation (RPI) to explain age-related variance in fracture toughness. Harvested from cadaveric femurs (62 human donors), single-edge notched beam (SENB) specimens of cortical bone underwent fracture toughness testing (R-curve method). NMR-derived bound water showed the strongest correlation with fracture toughness properties (r=0.63 for crack initiation, r=0.35 for crack growth, and r=0.45 for overall fracture toughness; p<0.01). Multivariate analyses indicated that the age-related decrease in different fracture toughness properties were best explained by a combination of NMR properties including pore water and RPI-derived tissue stiffness with age as a significant covariate (adjusted R2 = 53.3%, 23.9%, and 35.2% for crack initiation, crack growth, and overall toughness, respectively; p<0.001). These findings reflect the existence of many contributors to fracture toughness and emphasize the utility of a multimodal assessment of fracture resistance. Exploring the mechanistic origin of fracture toughness, glycation-mediated, non-enzymatic collagen crosslinks and intra-cortical porosity are possible determinants of bone fracture toughness and could explain the sensitivity of NMR to changes in fracture toughness. Assuming fracture toughness is clinically important to the ability of bone to resist fracture, our results suggest that improvements in fracture risk assessment could potentially be achieved by accounting for water distribution (quantitative ultrashort echo-time magnetic resonance imaging) and by a local measure of tissue resistance to indentation (RPI).